The drug manufacturer, GlaxoSmithKline, announced a discontinuation of brand Flovent HFA (fluticasone propionate inhalation aerosol) and Flovent Diskus (fluticasone propionate inhalation powder) products on December 31, 2023. Texas Medicaid anticipates the supply of these products will be unavailable beginning in early 2024. These drugs are used to treat and prevent asthma.
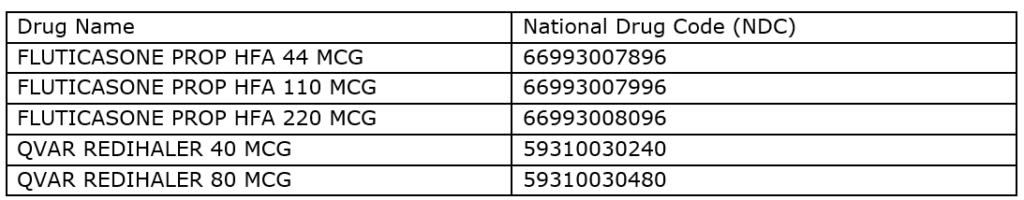
To address a shortage of these drugs, Texas Medicaid will make generic fluticasone HFA and Qvar Redihaler, a similar drug, available to Texas Medicaid clients without prior authorization. Also, brand name Flovent HFA and Flovent Diskus products will continue to be available on the Texas Medicaid formulary until the existing supply of these drugs runs out.
The Texas Health and Human Services Commission will cover the following drugs without requiring prior authorization starting December 15.

Recent Comments